Recently, the research team of Prof. Lianhui Wang and Zhimin Luo from State Key Laboratory for Organic Electronics and Information Displays, School of Chemistry and Life Sciences in Nanjing University of Posts and Telecommunications, along with Professor Yunlu Dai's team from the University of Macau, achieves new progress in the field of tumor radiotherapy and immunotherapy. Their findings, titled Two-dimensional coordination risedronate-manganese nanobelts as adjuvant for cancer radiotherapy and immunotherapy, are published in the international academic journal, Nature Communications. Prof. Lianhui Wang, Zhimin Luo along with Prof. Dai, are co-corresponding authors of this paper. Young faculty member Zhusheng Huang and master's student Shiqian Huang are co-first authors. Prof. Lixing Weng provides significant support for the genome-wide RNA transcriptome analysis in this paper.

Radiation therapy-induced tumor damage can be viewed as a promising opportunity to establish endogenous in situ vaccines. However, the efficacy of in situ cancer vaccination (ISCV) induced by radiation therapy (RT) is very weak and often insufficient to elicit systemic anti-tumor immunity. Therefore, Prof. Lianhui Wang and Zhimin Luo's team develops two-dimensional risedronate-manganese nanobelts (RMn-NBs) based on the clinical osteoporosis drug (risedronate) and trace element (manganese ions) as an immunoadjuvant for radiotherapy, by using a drug self-assembly delivery strategy to enhance the tumor in situ vaccine effect induced by radiotherapy. RMn-NBs exhibit excellent T2 magnetic resonance imaging performance and enhanced Fenton-like catalytic activity, effectively inducing immunogenic cell death in tumor cells. Additionally, RMn-NBs significantly inhibit the HIF-1α/VEGF pathway, and activate the cGAS/STING pathway to promote the secretion of type I interferons, thereby strengthening the ISCV effect induced by radiotherapy and enhancing immune checkpoint blockade therapies for both primary and metastatic tumors. As a nanoadjuvant for radiotherapy, RMn-NBs demonstrate good biocompatibility and therapeutic efficacy, offering promising applications for cancer radiotherapy and immunotherapy.
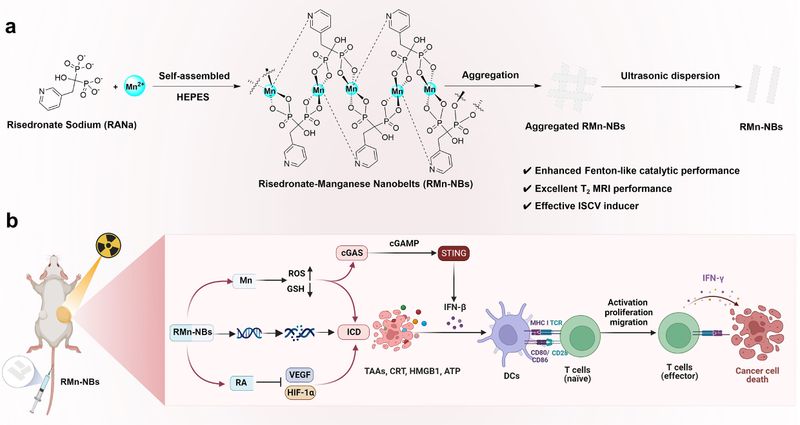
Figure. Two-dimensional coordination risedronate-manganese nanobelts as adjuvant for cancer radiotherapy and immunotherapy
The innovative use of existing drugs and the drug self-assembly delivery strategy provide new insights for radiotherapy and clinical translation of immunotherapy. This work is supported by the Natural Science Foundation of Jiangsu Province-Major Project, the National Natural Science Foundation of China, Macao Young Scholars Program, Natural Science Key Fund for Universities in Jiangsu Province, General project of natural science research in colleges and universities of Jiangsu Province, Natural Science Research Start up Foundation of Recruiting Talents of Nanjing University of Posts and Telecommunications.
(Written by Zhusheng Huang, Initially Reviewed by Zhimin Luo, Zuqin Qiao and Xiubin Dai, Edited by Cunhong Wang, Reviewed by Feng Zhang)



